Baking sodapowder is a white crystalline powder. 2 NaHCO 3 Na 2 CO 3 CO 2 H 2 O.
These manufacturers are affiliated with major corporations such as PepsiCo or the Coca-Cola Company which supply them with the often secret formula to the beverage.
Manufacturing process of baking soda. Manufacturing Process - Baking soda. Baking sodapowder is a white crystalline powder. It comes from what is known now as Soda ash.
Soda Ash is only obtained from mining an ore called trona or through the Solvay process. Trona ore is mined at 1500 feet below the surface. An illustration of the baking soda manufacturing process.
A key step in the process occurs in the carbonating tower. Next the dried crystals of sodium bicarbonate are separated into various grades by particle size. Each grade goes to a holding bin wherein atmosphere carbon.
Manufacturing Process The manufacture of baking powder begins with the production of sodium carbonate. In this process ammonia and carbon. The bicarbonate crystals are filtered out using vacuum filters or centrifuges.
They are then washed with water to remove. The soda ash is dissolved. Baking soda is commercially produced through the following process.
Sodium carbonate solution is treated with carbon dioxide to form a slurrysuspension. The suspension is separated from the liquid producing a cake. The cake is washed in a bicarbonate solution.
Soda ash can be made artificially using the Solvay process or it can be made from trona ore. Trona ore must be mined for 1500 feet down then it must transported to a variety of processing plants which it gets turned into soda ash. Making baking powder 1.
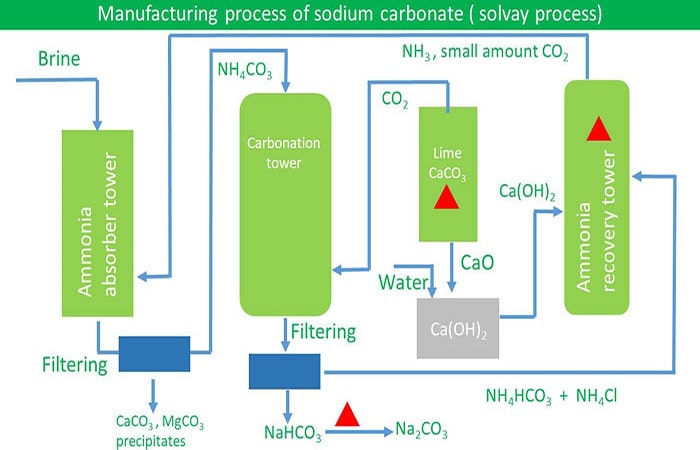
The soda ash is put into a centrifuge which separates the liquid from the crystals. Sodium carbonate manufacturing process solvay process Washing soda soda ash baking soda. Solvay process steps or ammonia-soda process.
Solvay process has five main steps on sodium carbonate manufacturing. Requirements to build a solvay process plant. Ability of getting limestone easily.
The important chemical reaction that is used in the production of baking soda and sodium carbonate is. CO 2 H 2 O NH 3 NaCl NaHCO 3 NH 4 Cl. 2 NaHCO 3 Na 2 CO 3 CO 2 H 2 O.
Carbon dioxide produced is recycled to produce NaHCO 3. Soda is usually made by independent manufacturers at canning and bottling plants most of which are strategically located near major markets. These manufacturers are affiliated with major corporations such as PepsiCo or the Coca-Cola Company which supply them with the often secret formula to the beverage.
The Solvay Process also known as the ammonia-soda process developed in 1861 is the worlds major industrial process for the production of sodium carbonate NaCO 3 or soda ash. In 1861 after realizing the polluting impacts of the Leblanc Process Belgian industrial chemist Ernest Solvay rediscovered and perfected Augustin Fresnels reaction. Baking Powder Production Process.
The manufacturing process is standardized and simple. It is advisable to source the manufacturing technology from the Government research center. Here we are providing the basic steps.
First of all you will need to procure the various ingredients. Baking Powder Production Process. The manufacturing process is easy and simple.
It is advised to source the manufacturing technology from the Government research center. The Basic Step Includes. 1Procure the various ingredients.
Sodium bicarbonate is mainly produced by the Solvay process. Solvay process is sodium chloride ammonia and carbon dioxide reaction in water. Sodium bicarbonate can be prepared by carbon dioxide reacts with the sodium hydroxide aqueous solution.
According to documents from Church and Dwight manufacturer of Arm Hammer The sodium bicarbonate manufacturing process begins with the dissolution of sodium carbonate commonly called soda ash or washing soda in purified water. Carbon dioxide is bubbled through the soda ash solution and baking soda is formed. Commercial quantities of baking soda are also produced by a similar method.
Soda ash mined in the form of the ore trona is dissolved in water and treated with carbon dioxide. Sodium bicarbonate precipitates as a solid from this solution.
